
Professor S. Egginton
- Position: Visiting Professor of Exercise Sciences
- Areas of expertise: physiology (cardiovascular, respiratory, skeletal muscle); angiogenesis; peripheral oxygen transport; microcirculation; exercise; thermal acclimation
- Email: S.Egginton@leeds.ac.uk
- Phone: +44(0)113 343 2264
- Website: Antarctic blog | Cold blood lecture | Googlescholar | Researchgate | ORCID | White Rose
Profile
BSc(Hons) Zoology, University of Wales, Bangor; PhD Physiology, University of St. Andrews, Fife; Postdoctoral Fellow at University of Maine, Orono (USA) and University of Birmingham; Lecturer, Senior Lecturer and Reader in Cardiovascular Physiology at University of Birmingham
Research interests
Integrative cardiorespiratory and exercise physiology.
My group studies the mechanisms of blood vessel growth, using physiological remodelling to devise targetted therapeutic repair of a pathologically damaged microcirculation, and interactions between the cardiovascular and respiratory systems in exercise and hypothermia, and how deleterious effects may be ameliorated e.g. using electrical stimulation or therapeutic bradycardia.
The multidisciplinary approach ranges from mathematical modelling and bioinformatic network analysis, through cell and molecular assays, to in vivo tissue performance and integrative function in a range of animal species and man.
These experiments explore the limits to physiological adaptation, and the origins of human morbidity.
1) Icefish—one of nature’s great curiosities
In April 2015 a group of US, UK, Canadian and Swedish researchers including myself headed to the Antarctic Peninsula as part of a three year investigation of the icefish—one of nature’s great curiosities.
For more information you can read a blog of the expedition at http://www.fbs.leeds.ac.uk/blogs/antarctic/
2) Angiogenesis
The Angiogenesis Research Group is studying the mechanisms underlying growth of blood vessels in skeletal and cardiac muscle, with a particular emphasis on determining how mechanical factors (i.e. the local physical environment of endothelial cells) may initiate and control the proliferation of capillaries during in vivo angiogenesis. Currently under investigation is how increased blood flow (elevated shear stress) and muscle overload (increased longitudinal strain) lead to different patterns of growth originating at the inner (luminal) and outer (abluminal) surfaces, respectively.
The combination of local factors invoked during muscle activity is compared with those seen during alterations in the external environment (e.g. hypoxia, hypothermia), including the differential influence of humoral and perivascular signals. These data are essential for the development of angiotherapies to promote regulated vascular growth or control vascular rarefaction, as required. This work has widespread implications - some estimates suggest 80% of NHS medical burden could benefit from targetted angiotherapy.
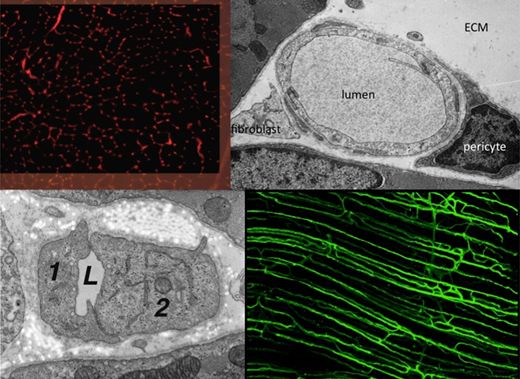
Important developments include the observations that capillary growth can be induced with or without breakage of the basement membrane, endothelial proliferation may precede the formation of sprouts, the lack of involvement of FGF, and that VEGF, NO and metalloproteinases may differentially regulate physiological angiogenesis. These findings do not conform to the patterns established for pathological or in vitro models of angiogenesis.
Further studies to elucidate this distinction include the role of stromal cells (pericytes, fibroblasts, macrophages) in controlling angiogenesis, and the link between small and large vessel growth. Exciting new avenues for angiotherapy being explored include manipulation of the HIF transcription pathway, the potential role for stem cells in treatment of muscle ischaemia (using endothelial progenitors), and how mobilisation of other blood components (platelets, leukocytes) may affect the angiogenic response to physiological or pathological stimuli.
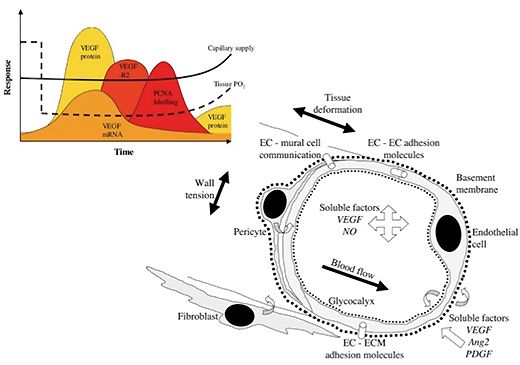
3) Oxygen transport
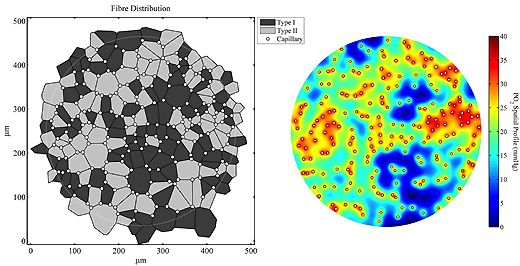
The responses to impaired blood flow or increased activity are used to examine how oxygen supply and demand are balanced. Analysis of capillary distribution within heterogeneous tissue (e.g. muscles with different fibre types) utilises morphometric quantification of capillary supply among fibres in terms of 'supply equivalents'.
Scaling of capillary supply with respect to fibre size is the usual pattern observed in skeletal muscle of all vertebrates, and this basic growth process may be modified to a limited extent with training or under pathological conditions (see above). This work identifies the need for tight spatial organisation of adaptive angiogenesis. Complimentary to the extracellular diffusion distances described in this manner, changes to intracellular oxygen tension are examined using mathematical models to examine the relative efficiency of changes in different components within the pathway of oxygen transport to tissue.
Bioinformatic analysis of complex diseases, those with central and peripheral components involving impaired oxygen transport, is used to explore the possibility of better targetted treatment for established co-morbidities (e.g. heart failure, respiratory disease).
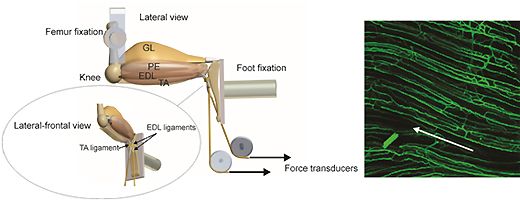
4) Muscle plasticity
The metabolic and ultrastructural responses of skeletal muscle to chronic reduction in blood flow or increased activity are followed in order to identify the range of strategies available to balance nutrient supply and demand. Work on chronic ischaemia is continuing to examine the apparent dissociation between structural and functional damage to the endothelium, and impairment of vascular reactivity liked with NO production.
Glycolytic and oxidative muscles respond differently to similar stimuli, although metabolic and structural indices of aerobic capacity are closely related in most, but not all, cases (e.g. capillary supply and oxidative capacity may be varied independently, and localised interventions may have significant remote effects).
Adaptations examined include myopathic and training responses in humans; electrical stimulation and ischaemia in mammals; steroid and overload hypertrophy in mammals; migratory fitness in a range of vertebrate species.
5) Cardiorespiratory control
How well the cardiovascular system copes with reduced core temperature has major implications for survival in all animals, and use of clinically invoked hypothermia in humans. Using a specially designed environmental chamber (with photoperiod and temperature control), the strategies adopted by species that adapt to a cold environment are compared with the more limited tolerance of other mammals (including man).
The cardiovascular response of hibernators to hypothermia examines angiogenic potential, vascular reactivity and cardiac autonomic control mechanisms; this has required development of new software (for power spectral analysis) and hardware (data loggers for long term storage), with potentially broad application. Ectotherms from polar & tropical environments provide additional insights into the physiological limits to life on the edge from an evolutionary perspective.
Identifying the preserved or ancestral mechanisms will define the most robust, and therefore likely profitable, avenues for therapeutic intervention.
Qualifications
- BSc (Bangor)
- PhD (St Andrews)
- DSc (Birmingham)
Professional memberships
- Fellow of The Royal Society of Biology
- Fellow of The Physiological Society
Research groups and institutes
- Sport and Exercise Sciences

