Rescuing ribosomes and bewildering bear hugs: inside a critical biological process
A group of scientists from the University of Leeds and the University of Dundee have discovered new knowledge in how ribosomes – the key machinery that makes proteins for our cells - are rescued.
In the paper, The UFM1 E3 ligase recognizes and releases 60S ribosomes from ER translocons, scientists have revealed greater detail into the process of UFMylation – when a protein ‘rescuer’, UREL, modifies faulty ribosomes triggering a recycling process.
Ribosome UFMylation has caught the attention of scientists in recent years for its ability to help maintain normal protein production and cellular function. However, mechanistic insights on this fundamental process are lacking which is a problem if we want to develop new treatments for diseases that work by disrupting these healthy cell processes.
“Using highly sophisticated imaging techniques here at Leeds, we’ve revealed this unique structure of UREL bound to the ribosome for the first time,” she added.
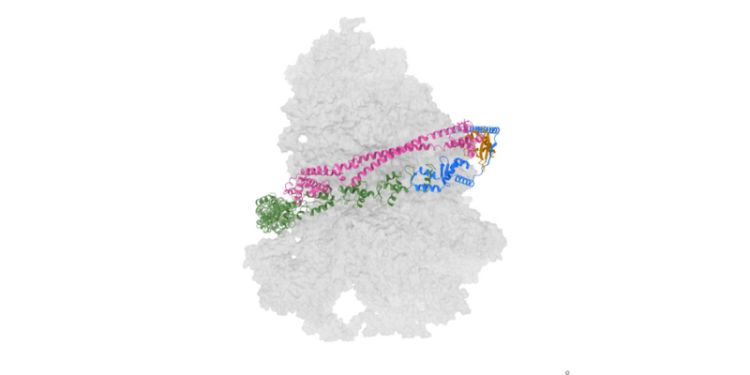
A critical biological process in action
The researchers used an imaging technique called cryo-electron microscopy (cryo-EM) to visualise the 3D structure of UREL bound to the ribosome with high-resolution detail.
This uncovered that UREL modifies malfunctioning ribosomes by wrapping itself around a ribosome – in a way similar to that of a bear hug. UREL then attaches a small protein, called UFM1, onto the ribosome which helps release the stalled ribosomes from the Endoplasmic Reticulum membrane (ER membrane), a part of the cell which produces proteins for the rest of the cell to function.
By releasing the malfunctioning ribosome, the cell prevents a toxic build-up of materials that can lead to various diseases such as neurodevelopmental disorders and skeletal abnormalities.
New treatments
Understanding the UFMylation process offers hope for treatments for diseases that develop through mutations in the UREL proteins and toxic build-up of stalled ribosomes.
I am excited that we have answered a fundamental and long-standing question in biology revealing an essential step for quality control pathways to function at the ER membrane. As mutations in this pathway cause neurodevelopmental disorders and skeletal abnormalities, we hope we can also begin to pave the way for new therapeutic approaches to help these patients.
An unexpected discovery
The way in which UREL wraps around the ribosome is due to the fact it is both a ‘reader’ and ‘writer’ of UFM1.
“In UFMylation biology, one way of differentiating the groups of proteins that interact with UFM1 is whether they’re a ‘writer’ or ‘reader’ of the UFM1 modification. A ‘writer’ adds UFM1 onto a target protein within our cells and a ‘reader’ is able to interpret this UFM1-modified target to act. These processes are fundamental for cells to carry out their day-to-day functions,” said Professor Elton Zeqiraj, Wellcome Trust Senior Research Fellow, School of Molecular and Cellular Biology.
We usually find that these proteins specialise in one function or the other so UREL being able to act as both a ‘writer’ and a ‘reader’ is very unusual. It appears UREL is quite the all-rounder.
The paper, The UFM1 E3 ligase recognizes and releases 60S ribosomes from ER translocons, which is funded by Wellcome Trust and the Medical Research Council, is published in Nature.




