Groundbreaking microscopy unlocks secrets of plant virus assembly
New research into how a plant virus assembles could lay the groundwork for future use to carry drugs into the human body.
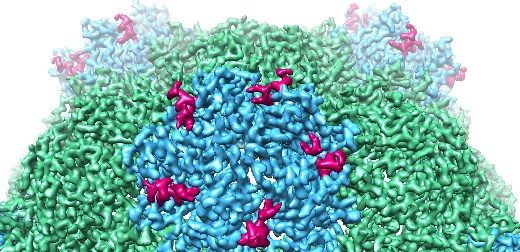
The study, by a team from the University of Leeds’ Astbury Centre for Structural Molecular Biology and the John Innes Centre in Norwich, describes the structure of an empty version of Cowpea Mosaic Virus (CPMV) and the molecular “glue” that allows the virus to build itself and encapsulate its genome.
The findings, published in the journal Nature Communications and based on revolutionary new electron microscopy, may be a crucial step to eventually allowing scientists to build custom versions of the virus that can carry medicines into the body and target disease.
Lead author Dr Neil Ranson, Associate Professor of Structural Molecular Biology at the University of Leeds, said: “To use Cowpea Mosaic Virus as a drug delivery vehicle, we need to understand how it puts itself together, and to do that we need to understand its structure in solution in very fine detail.
“Just a couple of years ago, that was impossible because we simply couldn’t see complex biological systems in the detail required. A new generation of electron microscopes, however, is revolutionising our ability to peer into the virus’ inner workings and understand how we might make it work for us.”
The Nature Communications paper investigates vital steps to understanding how safe, plant-based virus-like particles could be created in the future.
Dr Ranson said: “The aim of our project is to understand how the virus can put itself together from very simple building blocks. If we understand that properly, we may be able to efficiently make the virus package drugs, and then target them towards specific places or diseases in the human body, such as cancer cells.
“Plant viruses are ideal for such work – they are a huge evolutionary distance from us. You can’t catch plant viruses. Our paper shows the structure of the empty virus shell in unprecedented detail, including a part of the protein that is essential for assembly but has never been seen before. The virus-like particles, which were made by our collaborators at the John Innes Centre in Norwich, have no genome and therefore no ability to reproduce themselves or mutate,” Dr Ranson said.
“We are left with elegant, highly efficient and stable structures that have evolved to a level of perfection that it is currently impossible for man-made designs to rival, and these could be a major asset in developing targeted medicines. We could in the future change the sequences on their protein shells and retarget them at the diseases we want to hit.”
The paper is a product of a revolution in electron microscopy—dubbed the “resolution revolution”—that is transforming the level of detail at which structural biologists can work. It includes some of the most detailed electron microscope structures of protein complexes yet published, and these form the basis of a detailed analysis of how the CPMV virus builds itself.
The researchers show how the virus constructs a highly symmetrical, protein shell from five-sided “pentons” each built from five copies of a protein subunit. At the heart of the assembly process is a segment of a key protein – the C-terminus of the small coat protein subunit – that acts as a dab of molecular glue to hold the pentons together as the virus’ outer structure is built.
The C-terminus is also essential for the virus to package its genes, but it is cleaved from the virus when it has done its job. This has made it impossible to observe using other structural techniques such as x-ray crystallography.
Dr Emma Hesketh, a University of Leeds Research Fellow and the first author of the paper in Nature Communications, said: “The basic unit is very simple, so the virus only needs a very small amount of information to make a large protein shell. Not only is it very efficient but CPMV is known for building a very stable structure that doesn’t break down easily. We need that stability if these structures are going to survive drug manufacture and be introduced into the human body.”
“The new electron microscopes used in this study allowed us to see the segment in detail and understand its real role,” Dr Hesketh said.
The team used new-generation 300-kilovolt electron microscopes equipped with direct electron-detecting cameras at the Medical Research Council (MRC) Laboratory of Molecular Biology in Cambridge. The microscopes are capable of more than 130,000-times magnification. Two of the latest generation of this type of electron microscope are part of a £17 million investment in the new Astbury Biostructure Laboratory and are due to be installed at the University of Leeds next year.
“This equipment is completely transforming the level of detail at which we can interact with molecules. The new microscopes have more power but are also more stable and have sensors that directly detect the electron beam, rather than indirectly detecting it with optical sensors as the previous generation did.
“In practice, that means that, for the first time, we are looking in atomic detail at the individual amino acids in complex biological systems. This opens the way to manipulating those amino acids and intervening in the function of molecules with unprecedented precision.”
The research was funded by the Biotechnology and Biological Sciences Research Council (BBSRC) as part of the project entitled “Untangling the processes of replication and encapsidation in Picornavirales” led by Prof. George Lomonossoff at the John Innes Centre.




